Comprehensive resources and coverage so you and your patients can focus on treatment
Administering Euflexxa
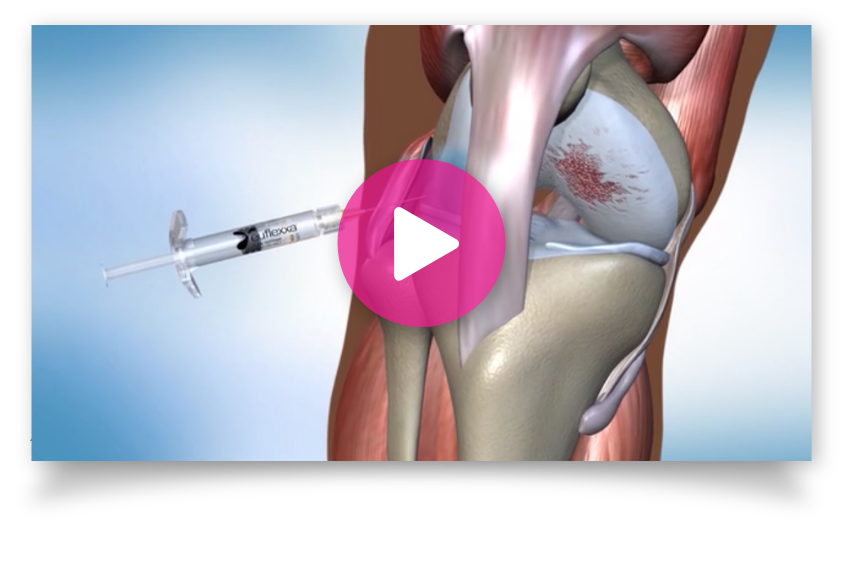
Office Materials

Brochure

Instructions
Dual-Acting Effects
Find out how EUFLEXXA may help your patients’ OA knee pain in two ways.
Discover the EffectsAccess
Most commercially insured patients can get EUFLEXXA under their medical benefits.
fewer access restrictions*
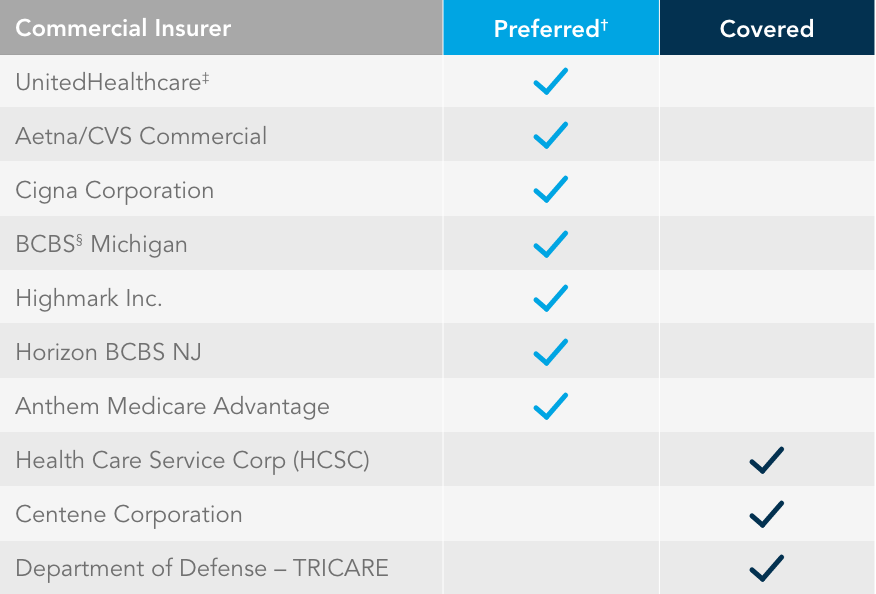
EUFLEXXA is covered under the pharmacy benefit by many insurers such as UnitedHealthcare, OptumRx, Express Scripts, and the Veterans Administration.
*Subject to change based on individual plan coverage. Check the member’s benefits for more details.

*Subject to change based on individual plan coverage. Check the member’s benefits for more details.
Euflexxa Solution Center
Benefits investigations are completed within 24 hours while you track progress online
For Solution Center questions call 1-866-383-5391 or fax 1-866-383-5392.
Patient Direct Program

Euflexxa Commitment
If your patient is not satisfied with their OA knee pain relief 10 weeks after their last EUFLEXXA injection, we offer up to a $100 refund for copay costs. The patient must have received 1 EUFLEXXA injection a week for 3 weeks between the eligibility period.
To receive the refund, we need the following information no later than 14 weeks after the patient’s last injection to process their refund:
- Completed refund form
- Explanation of Benefits for each injection from the insurance company
- Receipts for their EUFLEXXA copay payment
- Fax the documents to 1-866-383-5392
†Preferred access refers to favorable formulary status, medical policy, or fewer restrictions for EUFLEXXA.
‡Allowed for buy and bill.
§BCBS IL, BCBS MT, BCBS NM, BCBS OK, and BCBS TX.
¶The Solution Center will respond to each fax submission within 24 hours, notifying you of a patient’s coverage via the Benefits Investigation Report.
#The EUFLEXXA Solution Center does not file claims or appeal claims, and cannot guarantee that you will be successful in obtaining reimbursement. Third-party payment for medical products and services is affected by numerous factors, none of which can be anticipated or resolved by the Solution Center.
Dual-Acting Effects
Find out how EUFLEXXA may help your patients’ OA knee pain in two ways.
Discover the Effects- Rolling 12 month average of IQVIA claims data based on unique patients (December 2022).
- Data on file. Ferring Pharmaceuticals Inc. (December 2022).
