EUFLEXXA keeps you moving forward in 2 ways2-5

How is EUFLEXXA Different?
EUFLEXXA is the #1-prescribed HA injection that may help you enjoy the moments that matter by treating your OA knee pain in 2 ways.1-5
Lubricates & Cushions2-4
Reduces Inflammation & Pain2-5
in the body
With its high molecular weight and linear structure,
EUFLEXXA most closely resembles the natural, healthy HA in your knee2,6-11
Natural, Healthy HA
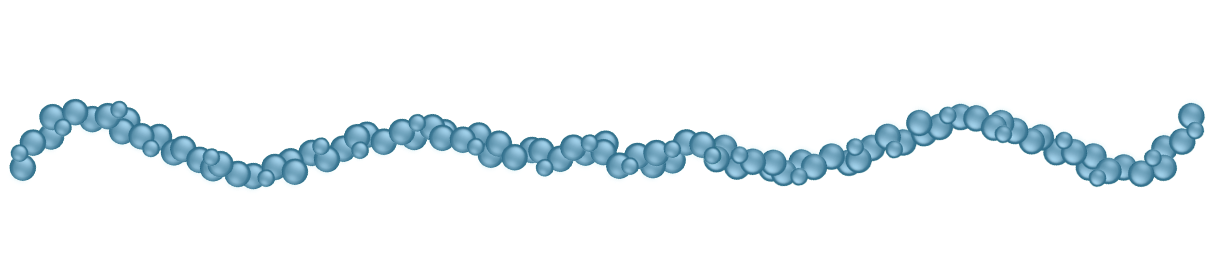
EUFLEXXA


Coverage & support
Learn about insurance coverage and the support you may be able to receive throughout treatment.
Get Support- Rolling 12 month average of IQVIA claims data based on unique patients (December 2022).
- EUFLEXXA [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.
- Altman R, et al. Anti-inflammatory effects of intra-articular hyaluronic acid: a systematic review. Cartilage. 2019;10(1):43-52.
- Altman RD, Manjoo A, Fierlinger A, et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskeletal Disorders. 2015;16:321.
- Yang C, Cao M, Liu H, et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem. 2012;287(51):43094-43107.
- Nicholls M, et al. A comparison between rheological properties of intra-articular hyaluronic acid preparations and reported human synovial fluid. Adv Ther. 2018;35:523-530.
- Orthovisc [package insert]. Woburn, MA: Anika Therapeutics, Inc; 2005.
- Supartz [package insert]. Durham, NC: Bioventus LLC; 2012.
- Gelsyn-3 [package insert]. Durham, NC: Bioventus LLC; 2016.
- Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc; 2011.
- Genvisc-850 [package insert]. Doylestown, PA: OrthogenRx; 2016.
